|
|

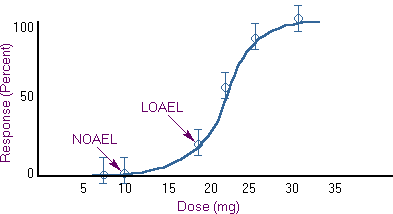
NOAEL and LOAEL
Two terms often encountered are No Observed Adverse Effect Level (NOAEL) and Low Observed Adverse Effect Level (LOAEL). They are the actual data points from human clinical or experimental animal studies.

 |
||
Sometimes the terms No Observed Effect Level (NOEL) and Lowest Observed Effect Level (LOEL) may also be found in the literature. NOELs and LOELs do not necessarily imply toxic or harmful effects and may be used to describe beneficial effects of chemicals as well.
The NOAEL, LOAEL, NOEL, and LOEL have great importance in the conduct of risk assessments.